Isomerism in Coordination Compounds
Isomerism in Coordination Compounds: Overview
This Topic covers sub-topics such as Optical Isomerism, Geometrical Isomerism, Stereoisomerism, Structural Isomerism, Isomerism in Coordination Compounds, Linkage Isomerism, Coordination Isomerism, Optically Active Compounds and, Ionization Isomerism
Important Questions on Isomerism in Coordination Compounds
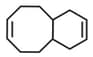
Number of geometrical isomer of given compound will be:
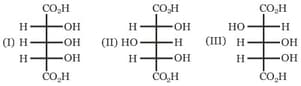
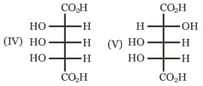
Which of the above compounds are enantiomers?
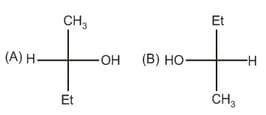
Relation between given pair is:
Among the following, a pair of resolvable configurational enantiomers is given by:
How many minimum no. of C-atoms are required for position and geometrical isomerism in alkene?
Which of the following compounds does exhibit stereoisomerism?
Which of the following has largest number of isomers?
(R is alkyl group, en is ethylediamine).
Which are the pairs of enantiomers and diastereomers from the following
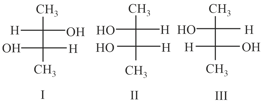
The complex(es), which can exhibit the type of isomerism shown by , is(are)
Which of the following complex has a possibility to exist as meridional isomer?
Which of the following can be represented as a meridional isomer ?
In 20th century, German scientist Werner succeeded in clarifying the structures of the five compounds consisting of platinum, chlorine, and ammonia. Some of the properties of these compounds are shown below in the table.
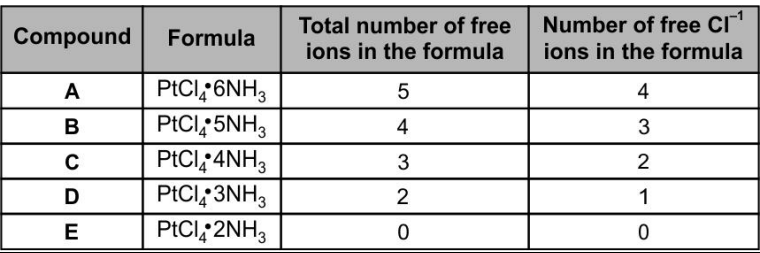
Which of the complexes formed for the compounds have structural isomers?
The complexhas two isomers whereas does not show geometrical isomerism and has no isomers why?
How many geometrical isomers are possible for complex ion?
The complexes and are
Among the complexes given below, the compound(s) that exhibit(s) optical isomerism is(are):
(i) cis- ;
(ii) trans- ;
(iii) cis- ;
(iv) trans-
Which among the following compound does not has enantiomeric pair?
Which isomerism is possible in hexa ammine cobalt (III) hexacyanido chromate (III) complex?
Which type of Isomerism in isomers and ?
Square planar complexes do not show optical isomerism.
